

In this article, we will learn about carbohydrate structure and properties of the three main carbohydrate classes: monosaccharides, oligosaccharides, and polysaccharides.
Carbohydrates are one of life’s most important classes of molecules. Specifically, carbohydrates are known for their functionality as units of energy, which is why “carbs” are an essential part of the human diet. Indeed, they play an important role in energy storage, in the form of polysaccharides, and energy expenditure, in the form of monosaccharides or “sugars”. Additionally, carbohydrates have structural functions as components of cell walls in plants and bacteria. Carbohydrates also aid in cellular identification, as unique chains of sugars attached to a cell membrane provide a “fingerprint” that allows recognition of the type and species of the cell.
Their important functions in metabolism, structure, and identification are allowed by carbohydrate structure and properties. As we will see, carbohydrates have remarkable structural diversity.
Monosaccharides are the simplest carbohydrate molecules. In fact, monosaccharides are monomers of larger carbohydrates, meaning that they are the smallest unit of carbohydrate structure and properties, and form the building blocks of larger molecules. All monosaccharides have a chemical formula of C(n)H(2n)O(n), with a basic structure of an unbranching chain. When illustrated as a linear molecule (Fischer projection), monosaccharides have a carbonyl group attached to one carbon, while the other carbons have an alcohol group as a functional group.


Biochemists number the carbons of monosaccharides based on the position of the carbonyl, with the end closest to the carbonyl labeled as carbon 1.

Biochemists classify monosaccharides differently based on the location of their carbonyl group. Monosaccharides with their carbonyl at one end of the carbon chain are called aldoses, referencing the aldehyde group formed by the carbonyl. Contrastingly, monosaccharides with their carbonyl on an internal carbon are called ketoses, referencing the resultant ketone.


Between monosaccharides with the same number of carbons, aldoses and ketoses are what chemists call “constitutional isomers” of one another. This means that they have the same chemical formula, but different connectivity between their atoms.
Additionally, many monosaccharides are enantiomers, or mirror images, of one another. Biochemists use the letters D and L to differentiate between monosaccharide enantiomers. In the Fischer projection, the designation of D versus L depends on the direction of the hydroxyl group of the chiral carbon furthest from the carbonyl. Specifically, D sugars have the hydroxyl group pointing right, while L sugars have a left-pointing hydroxyl group. Interestingly, biological systems only use one enantiomer of each monosaccharide, with D confirmation.

Despite the usefulness of the Fischer projection, most five or six-monosaccharides generally have cyclical molecular structures, due to unique carbohydrate structure and properties. With an acid catalyst, monosaccharides spontaneously undergo a cyclization reaction, where the carbonyl group is attacked by the hydroxyl of carbon 4 or 5. This occurs in both aldoses and ketoses, resulting in a five or six-membered ring where one of the members is the oxygen from the attacking hydroxyl.

Biochemists call the cyclic form of monosaccharides their Haworth projection, a carbohydrate diagram used to further classify sugars. For instance, biochemists classify five membered rings as furanoses and six-membered rings as pyranoses.
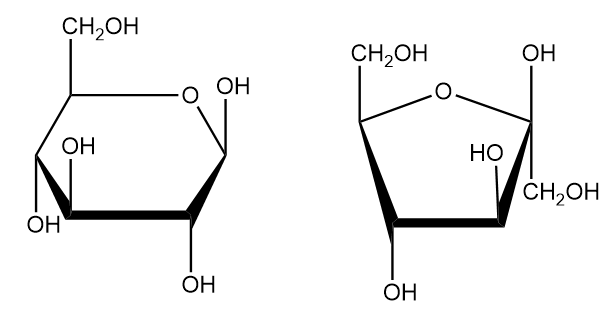
Interestingly, one unique property of carbohydrates is that all monosaccharides with greater than five carbons can form either furanoses or pyranoses. However, pyranoses are more common and stable, as six-membered rings generally always have less ring strain than five-membered rings.
Additionally, monosaccharide cyclization results in the carbonyl-carrying carbon becoming chiral. Two chiral positions of the carbon are possible from cyclization, and biochemists use the term “anomer” to differentiate between monosaccharides that only differ with respect to these positions. Accordingly, biochemists call the carbon that carries the carbonyl the “anomeric carbon.”
The terms “alpha” and “beta” describe these two isomers. Beta isomers have their anomeric hydroxyl group pointing in the same direction as carbon 6, while the alpha isomer has its anomeric hydroxyl group pointing in the opposite direction.
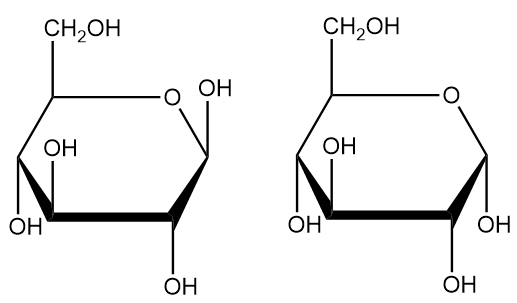
In line with other carbonyl-addition reactions, cyclization generally doesn’t favor a particular chiral position, so organisms generally have both anomers. However, anomers of some monosaccharides are more stable than others. For instance, beta-glucose tends to have more stability than alpha-glucose.
Let’s look at some monosaccharides that serve biological functions




Often, a few monosaccharides will agglomerate into structures called oligosaccharides. Many oligosaccharides only have two monosaccharides, called disaccharides, which is the main carbohydrate in plant syrup (sucrose), milk (lactose), and malt sugar (maltose).

Other common oligosaccharide structures include the branching chains that attach to membrane proteins or lipids on the outside of cells. The sequence and chemistry of these carbohydrate chains tends to be unique to each cell type, allowing for cellular identification in eukaryotic organisms.

Monosaccharides attach to one another using glycosidic linkages, which involves an oxygen atom linking two carbohydrate rings. Glycosidic linkage formation is a kind of condensation reaction, where the hydrogen of a hydroxyl group on one sugar and the entire hydroxyl group of the other sugar are lost, forming water as a byproduct. Like cyclization, this also involves an acid catalyst.

Additionally, every glycosidic linkage involves at least one anomeric carbon. The chirality of the anomeric carbon involved determines whether the glycosidic linkage classifies as “alpha” or “beta.” For instance, lactose always has a beta glycosidic linkage, because the anomeric carbon of beta galactose always forms the linkage with carbon 4 of glucose.
Conversely, glycosidic linkages break through hydrolysis reactions, which are essentially condensations in reverse.
Additionally, monosaccharides can form large polymeric structures called polysaccharides. Often, organisms use polysaccharides for energy storage or structural stability.
For energy storage, animals use a polysaccharide called glycogen while plants use one called starch. Both glycogen and starch are composed entirely of alpha-glucose linked with mostly alpha-1,4 glycosidic linkages, which are between carbons 1 and 4. These linkages form helical chains of glucose. Occasionally, alpha-1,6 linkages appear in the chains, which results in chain branching.

Glycogen tends to branch every 8-10 glucoses, while amylopectin, the branching component of starch, branches every 12-20 glucoses. Also, starch has a component called amylase, which forms simple, unbranched helices.
For structural stability, plants use the polysaccharide cellulose. Unlike starch and glycogen, cellulose uses beta-glucose and beta-1,4 linkages. Also, each alternating glucose has a flipped orientation which results in a straight chain, rather than a helix. To form a cell wall, glucose chains are laid beside one another, forming cellulose sheets, which are strongly held together by hydrogen bonds.

Other cell wall-forming polysaccharides include chitin, found in fungi, and peptidoglycan, found in bacteria. Both of these polysaccharides use chemically modified alpha-glucoses and beta-1,4 linkages.